
Aerial view of Ports of Los Angeles (left) and Long Beach (right). Source: Hyfen, CC BY-SA 3.0 via Wikimedia Commons
13. New Maritime Fuels to Reduce Greenhouse Gases and Air Pollution
Author: Dr. James A. Fawcett, USC Sea Grant Maritime Policy Specialist/Extension Director (retired)
Media Contact: Leah Shore / lshore@usc.edu / (213)-740-1960
Beginning in 2006 and in cooperation with one another, the two huge seaports of Los Angeles and Long Beach formed a unique partnership (between competitors and with the approval of the federal government) to improve ambient air quality in the Los Angeles air basin. Named the Clean Air Action Plan (CAAP),[1] It addressed mainly diesel engines; these are the engines that produce air pollutants that are routinely trapped in the Los Angeles air basin and contribute to respiratory problems and diseases.
The problem of reduced air quality is primarily due to the fuel used by these large engines but also due to the nature of diesel engines, which often produce soot in very small particles known as diesel particulate matter (DPM) and described by the size of the particles as PM10 (particles of 10 microns) or PM2.5 (particles of 2.5 microns). However, the engines also produce smog products such as oxides of nitrogen (NOx) and sulfur dioxide (SO2) that makes air pollution look brown, as well as carbon monoxide (CO) and large quantities of carbon dioxide (CO2), a greenhouse gas that contributes to global warming. These five (in addition to lead) are called “criteria air pollutants” by the U.S. Environmental Protection Agency (EPA) because they cause smog, acid rain, and other respiratory hazards.[2]
With the encouragement of the South Coast Air Quality Management District, the two ports acknowledged the importance of a CAAP and realized in the aughts that they needed to act responsibly to reduce their own contributions to air quality in the basin. What follows is an exploration of what has been done over the last 15 years and what may be the next steps in improving local basin air quality as well as reducing vessel contributions to global warming.
Shore Power
Among a number of remedial measures over the past 15 years, the CAAP has mandated that most of the cargo fleet “plugin” to shore power when ships are docked in either port. Since vast amounts of electricity are required when container ships are discharging and loading cargo, it can be generated in a much cleaner fashion by large power plants than by the diesel engines that ships use to generate auxiliary power onboard. For that reason, it makes sense to generate power off-site and for the ships themselves to use what amounts to a “maritime extension cord” to connect to the dock.[3] The U.S. EPA has estimated that use of shore power in ports can reduce CO2 emissions by 38%, NOX by 98%, and SO2 by 35% for passenger ships between 2,000 and 3,500 passengers.[4] This innovation has proved to be important as a model for both domestic and international seaports as they attempt to reduce their own impacts on air quality in cities with similar concerns over vessel-generated emissions. And, as proof of the concept, over the past 13 years of monitoring the results of the CAAP, the two ports have seen a general decline in the so-called criteria air pollutants that they monitor and report to the U.S. EPA under the Clean Air Act.[5]
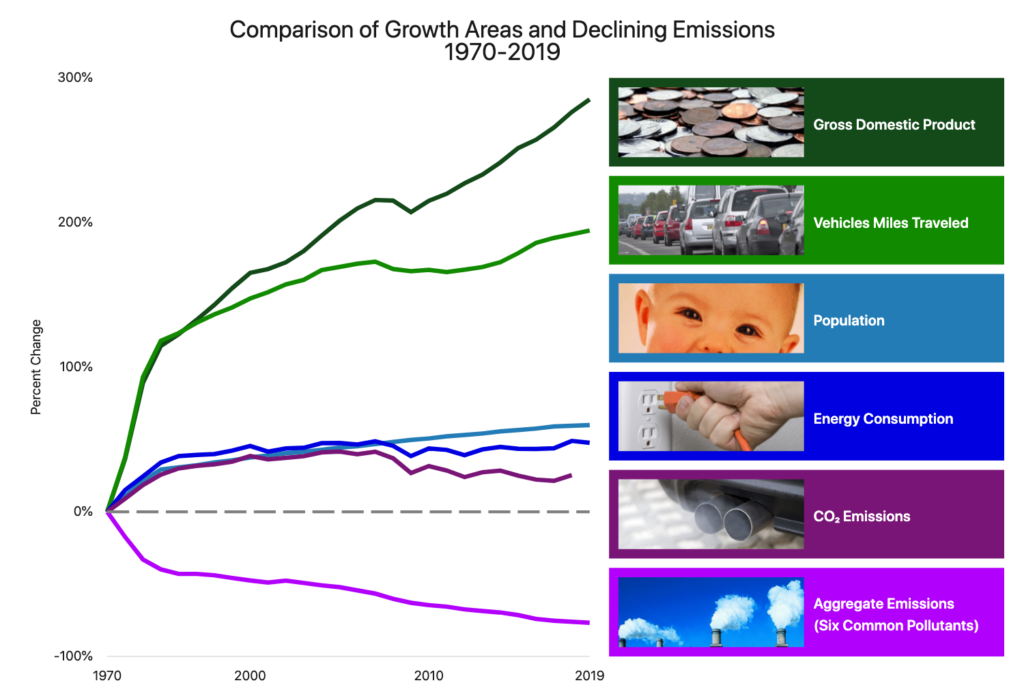
Climate Change and Diesel
Concern over global climate change has motivated governments globally to turn their lenses on the shipping industry and ask what can be done to improve the performance of the ubiquitous diesel engine. In other words, is there a workable alternative to diesel? Over the years, innovations have been made in managing the types of diesel fuel. There are also techniques for cleaning or “scrubbing” the emission products of exhaust, what is known as “stack gas,” to reduce or eliminate harmful waste products.
Recently, the International Maritime Organization (IMO), the UN agency that sets standards for international shipping, mandated a reduction in the sulfur content of diesel fuel worldwide to further combat the global effects of these emission products. But, only so much tinkering can be done with a fuel that is a complex mixture of hydrocarbons which, when oxidized to produce power, inevitably produces pernicious carbon-based waste products. Thus, scientists and the shipping industry have looked for alternatives to petroleum fuels.
Finding Alternatives
Alternative fuels first must be evaluated in terms of energy density because the viability of any alternative depends upon whether the vessel can devote enough space onboard for the fuel as well as revenue cargo. In other words, fuels take up space and contribute weight on a ship that has limited space and a weight capacity. In addition, the cost of the fuel per unit of volume is always an important consideration that determines economic viability. (Independent of its waste products, the more powerful fuel for an internal combustion engine will be the fuel that has the highest energy density.)
Unfortunately, many of the high energy density fuels also come with an array of waste or emission products, especially petroleum fuels. Capturing the pollutants is usually expensive or the alternative fuel has a much lower energy density, so for an equivalent amount of power, a vessel must carry a greater quantity of the fuel. And, pollution from these traditional fuels is no longer cost-free to the operator of the vessel.
To be acceptable, alternative fuels should reduce emission contaminants found in the exhaust of large-scale diesel engines, products that exacerbate global warming, respiratory disease, and damage air quality. But, since diesel engines were developed prior to our concerns over the environmental damage their exhaust can generate, our efforts at this point are an attempt to modify the existing mechanism and chemistry of that method of producing power rather than instituting a paradigm change. Hence alternative fuels will be a cleaner, but not perfect, alternative until an even cleaner means of generating power emerges.
So what are the candidate alternative fuels that have shown promise for the marine trades? The three that have garnered the most attention are ammonia, methanol, and hydrogen; we’ll explore each in turn.
Ammonia
Although we often think of ammonia as a cleaning agent, household ammonia is actually a weak concentration of ammonium hydroxide (NH4OH), an alkali solution that helps lift grease from surfaces. Worldwide, it is a major commodity chemical used in several industries, notably to manufacture fertilizers. The ammonia in the form of fuel is NH3 —anhydrous ammonia–a liquid with a boiling point of -33.3o C. It is commonly stored in pressurized containers in its liquid form and requires special conditions for storage and handling.
Just as a quick reminder about the basics of chemistry, energy is stored in the bonds between atoms. Certain chemicals make great fuels because when they are heated enough (by burning), their atoms break apart and release the energy stored between them. We use that energy to generate heat or power. Burning petroleum, a complex mixture of hydrocarbons, produces energy, but also vast amounts of CO2. If our objective is to reduce global warming, we want to find fuels that do not release CO2 as a byproduct. You may have noticed that the chemical formula for ammonia (NH3) contains no carbon (C), so burning it would not produce CO2. Ammonia burns because a diesel engine operates at a high enough temperature at which the hydrogen (H) and nitrogen (N) bonds can be broken.
Ammonia is a candidate because of its energy density and its lack of carbon. However, it has disadvantages as well. First, ammonia is both caustic and is classified as an “extremely hazardous substance” in the U.S.,[6] thus it must be handled very carefully when refueling. Also, careful maintenance must be exercised to prevent leakage, especially in engineering spaces where leaked gaseous ammonia could injure the vessel’s crew. More challenging is the chemistry of ammonia because, under high heat conditions, the nitrogen in ammonia plus the nitrogen content of ambient air can combine to produce large quantities of oxides of nitrogen (NOX), gases classified (as noted above) by the U.S. EPA as one of its criteria air pollutants.
Nevertheless, ammonia is still considered especially viable because of its energy density. Writing in the Journal of Power Sources, Zamfirescu and Dincer[7] claim that ammonia is third only to gasoline and liquid petroleum gas in terms of its power potential. Moreover, it is relatively inexpensive when compared to petroleum. The Department of Agricultural and Consumer Economics at the University of Illinois reports an average price of Ammonia over the past decade of approximately $700/ton[8], which is less expensive compared to the 0.1% sulfur fuel (very low-sulfur fuel oil or VLSFO[9]) priced at $567/metric ton[10].

Methanol
Another alternative fuel that has been discussed for large-scale maritime diesel engines is methanol (CH3OH or sometimes written MeOH). It is a common industrial solvent that is contained in windshield washer fluid mixed with water, in a jell form as “Sterno” as an additive for both gasoline and diesel engines, and as a feedstock for some fuel cells. It is easily handled at room temperature as a non-viscous liquid.
In maritime use, it has a host of advantages. It is widely produced and used throughout the industrialized world, which would make it relatively available for ships in almost any port Because of its low viscosity (“stickiness”), like water, both refueling and pumping it aboard ship is convenient using existing piping systems. With a high energy density (18.6 MJ/kg), methanol is a strong competitor for storage (ammonia contains 20.1 MJ/kg) [11], and is easier to handle. It, too, is relatively inexpensive compared to the $567/metric ton value of VLSFO marine fuel (mentioned above); recently, Methanex, a large producer in the US, quoted a price for North America at $542/metric ton.[12]
With a few caveats, methanol is a “drop-in” fuel in gasoline and diesel engines. The first caveat is that it is hygroscopic, meaning that it absorbs water. For that reason, it can corrode metal parts, especially aluminum. Also, its “flashpoint” (the flashpoint of a chemical substance is the lowest temperature where enough fluid can evaporate to form a combustible concentration of gas) unfortunately is quite low at 52oF, considerably lower than other contemporary petroleum fuels whose flashpoints are at least 100oF and often much higher.[13] In practical terms, having a low flash point for fuel is an extreme fire risk for ships. Onboard a ship where fire is secondary only to sinking as a prime hazard, the low flash point of methanol makes fire prevention an urgent everyday concern for mariners.
Another caveat of methanol as a fuel is its toxicity to humans. Even very small quantities, when ingested, can cause very serious health consequences including blindness and death. While petroleum and ammonia fuels are also toxic, they are also easy to identify without instruments. However, care must be taken to isolate potable water onboard from any source of methanol because of their easy ability to mix without leaving a trace smell or visual clue.
Nevertheless, methanol is used by Stena Line ferries that ply the Baltic Sea with passengers, freight, and automobiles. The attraction is that this is an area identified by the IMO as an “emission control area” or ECA, and the use of methanol as a substitute for diesel fuel greatly reduces the exhaust products. According to a recent article in Maritime Executive, “…using methanol eliminates emissions of Sulphur and particulate matter almost entirely and nitrogen by 60 percent compared to traditional ship fuel.”[14]

Hydrogen
The final alternative fuel that we will discuss is hydrogen (H2), which, unlike methanol and ammonia, would be an enormous paradigm change when used in fuel cells[15] to produce electricity. Writing in the journal Energy and Environmental Science in 2018, Staffell, et al. writes, “Conventional internal combustion engines can be modified to run on pure hydrogen (‘HICEs’) and could see early deployment as they are substantially cheaper than fuel cells. However, hydrogen combustion is less efficient than a fuel cell and releases NOx, hence is not expected to play a significant long-term role in transport.”[16] Fuel cells, however, could provide auxiliary power for ocean transport, but hydrogen fuel requires cryogenic storage onboard and, writing in 2019, Staffell remarks that H2 prices “significantly below $7 per kg [would be] needed” for hydrogen to be economic. Thus, the challenge for hydrogen is threefold: price, storage and type of use, and safety whether direct burning or as a feedstock for maritime fuel cells.
Conclusion
The industrial nations of the world will continue seeking a means of reducing the carbon burden on the climate and the marine transportation industry. Historically, steamships were able to burn “residual” fuels in their boilers, the residue from the refining process, also called “black oil.” As ships changed from steam power to diesel, more refined—but still carbon-heavy—fuels became common. Now that climate change attenuation demands further reductions in CO2 emissions, scientists and engineers have looked for fuels that carry even less of a carbon burden; ammonia, methanol, and hydrogen have received the most attention. None is a “perfect” fuel, but with further research, experimentation, and pilot projects, the market and governments will eventually settle on a suitable substitute for petroleum. It is likely that one or more of these three will become the standard for the ocean-going fleet.
Acknowledgments
I want to express my sincere thanks to Dr. Robert Aniszfeld, Managing Director of the USC Loker Hydrocarbon Research Laboratory for his assistance in advising me on fuel chemistry.
References
[1] Port of Long Beach and Port of Los Angeles. (2017). Clean Air Action Plan 2017 Update. https://cleanairactionplan.org/documents/2017-clean-air-action-plan-update-fact-sheet-10-23-17.pdf/
[2] US EPA. (1970). National Ambient Air Quality Standards under the Clean Air Act (42 U.S.C. 7401 et. Seq.)
[3] As a reminder, the propulsion engines (so-called primer movers) of the oceangoing fleet are but a part of the diesel engine suite on these large vessels and they are shut down once the vessel is secured at the dock.
[4] Passenger ships were used as a standard because the sizes of container ships vary widely. US EPA Office of Transportation Air Quality. (2017). Shore Power Technology Assessments at US Ports. P. 43, table 24. (Percentages derived).
[5] Port of Long Beach. (2020). Air Quality Monitoring Program at the Port of Long Beach: Annual Summary Report, Calendar year 2019. Port of Los Angeles. (2020). Air Quality Monitoring Program at the Port of Los Angeles: Annual Summary Report, May 2019-April 2020.
[6] US Government Publishing Office. (1 July 2008 ed.). 40 CFR: Appendix A to Part 355—List of Extremely Hazardous Substances and Their Threshold Planning Quantities (PDF).
[7] Zimfirescu C. & Dincer, I. (2008). Using ammonia as a sustainable fuel. Journal of Power Sources, 185(2008), 459-465.
[8] Schnitkey, G., Swanson, K., Paulson, N. & Zulauf, C. (April 20, 2021). Fertilizer Prices for 2021 Production. Univ. of Illinois, Dept. of Agricultural and Consumer Economics. https://farmdocdaily.illinois.edu/2021/04/fertilizer-price-increases-for-2021-production.html.
[9] In the North American sulfur emission control area (SECA), which includes the U.S. and Canada, the emission standard is 0.1% sulfur as set by the International Maritime Organization (IMO). Globally, the standard is 0.5% sulfur for most other waters, therefore, prices for 0.1% VLSFO will be more expensive than for 0.5% VLSFO. https://nepis.epa.gov/Exe/ZyPDF.cgi/P100AU0I.PDF?Dockey=P100AU0I.PDF
[10] Price represents the Los Angeles/Long Beach location on June 21, 2021. Taken fromhttps://shipandbunker.com/prices/am/nampac/us-lax-la-long-beach
[11] Aniszfeld, R. (personal communication). Also see: https://www.wartsila.com/media/news/01-12-2020-what-does-an-ammonia-ready-vessel-look-like–2825961
[12] Methanex. (April 27, 2021). Methanex Posts regional contract methanol prices for North America, Europe and Asia. https://www.methanex.com/our-business/pricing
[13] Engineering ToolBox, (2005). Flash Points – Liquids. [online] Available at: https://www.engineeringtoolbox.com/flash-point-fuels-d_937.html. Accessed 05/24/21.
[14] Maritime Executive. (7 Apr 2021). Stena Line aims to cut CO2 emissions by 30% by 2030. https://maritime-executive.com/corporate/stena-line-aims-to-cut-co2-emissions-by-30-percent-by-2030
[15] Fuel cells are electrochemical devices that have been used, for example, on spacecraft to generate electricity by combining a fuel such as hydrogen with an oxidizer such as oxygen. The “cell” does this through a chemical reaction — not by burning the fuel — and is scalable depending upon the amount of electricity required
[16] Staffell, I., et al. (10 Dec 2018). The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci., 2019, 12, 463-491.