The EASC Regulatory Science: East Asian Perspectives series represents an exciting collaboration with colleagues at the Health Sciences Campus to cover a wide range of topics on health and wellness that are of vital interest to us today. Regulatory Science studies the regulatory requirements for biomedical products in the United States and elsewhere around the world and East Asia plays a prominent role in all facets of this area — as developers, manufacturers, and consumers of regulated products.
Past Events
Thursday, February 11, 2021
The next event in this series will focus on Traditional Chinese Medicine (TCM) and its historical significance. In most Western countries, TCM gets labeled as Complementary Alternative Medicine (CAM) and viewed as supplemental to conventional medical practices. This discussion opens dialogue on the current state of Eastern medicines in the context of Western regulations.
Tuesday, October 20, 2020
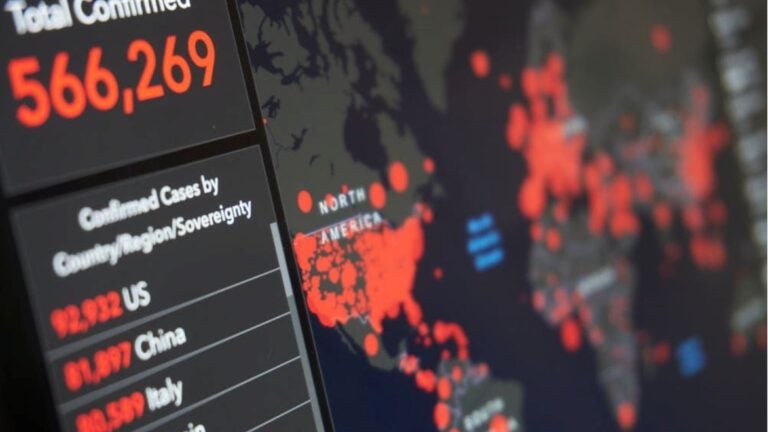
In our first webinar session for the series, we will focus on “Drugs in COVID Times” and examine the role of East Asia in global drug development. USC Professors Eunjoo Pacifici and Terry Church will weave history, current events, and future outlook of the region.
Tuesday, February 22, 2022
In this new era of rapid transitions, from onsite to remote and paper to digital platforms, we have an unprecedented opportunity to reimagine the way we approach many activities, including how new therapeutics are developed for a global population. Now more than ever, topics like regulatory convergence and harmonization, decentralized clinical trials, artificial intelligence, mobile health, and remote data capture are explored to reduce duplication of efforts, reuse data from one jurisdiction for another, and include historically under-represented populations in the clinical trial process. The seminar will provide an overview of this dynamic topic with a special regional focus on East Asia.