
Amidst an intricate setup of cables and advanced testing equipment, 2024 Wrigley Institute Graduate Fellow Yeshvi Tomar works to advance battery technology. (Nick Neumann/USC Wrigley Institute)
Harnessing the Power of Iron: A Promising Future for Clean Energy Storage with Iron-Air Batteries
Did you know? Almost all energy on Earth–about 130,000 TW–continuously comes from the sun, which is expected to last for another 4.5 billion years. That’s a huge amount of energy! Humanity doesn’t have an energy problem per se, but rather an energy storage and carrier problem.
Currently, the world economy is heavily dependent on fossil fuels which constitute up to 80% of world energy demands. Fossil fuels are non-renewable energy sources formed from the remains of prehistoric organisms buried in the earth’s crust over millions of years. These fossil fuels, which took nature eons to produce, can be classified as fossilized sunshine. They include coal, oil, and natural gas, which contain carbon and hydrogen. When burned, fossil fuels release large amounts of carbon dioxide, a greenhouse gas, into the atmosphere. This contributes to global warming, as greenhouse gases trap heat. The average global temperature has already risen by 1°C. Therefore, it is imperative to shift our energy needs to cleaner and safer sources. The solution to this challenge lies in the adoption of renewable energy.
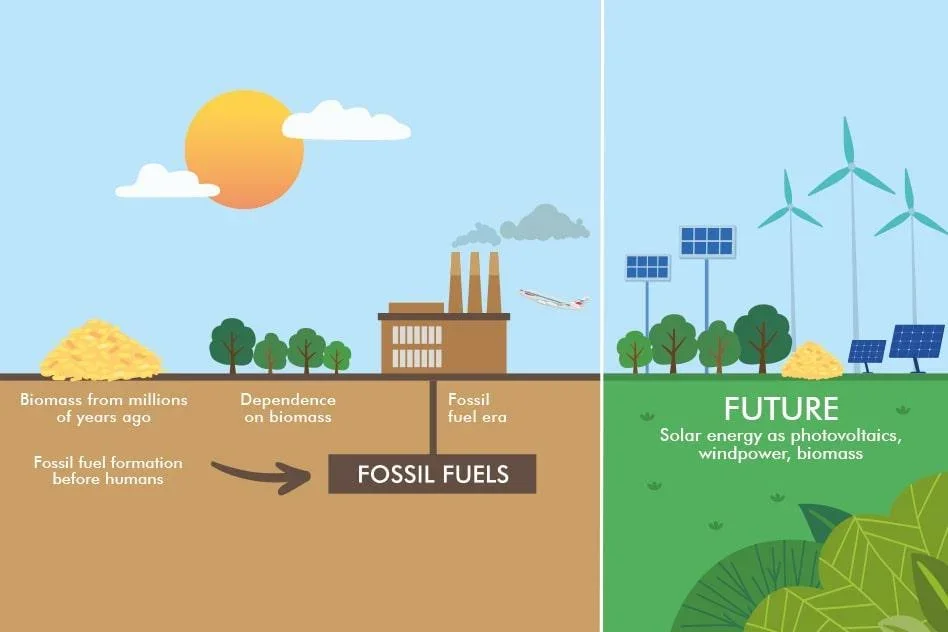
Some renewable energy sources depend heavily on natural forces and weather conditions, and are intermittent by nature. To utilize these sources effectively, they need to be coupled with energy storage devices. These devices store energy from the natural source when it is available and release energy according to the demands.
Currently, the global battery grid storage market is dominated by lithium-ion and lead-acid rechargeable batteries, which account for approximately 96% of the market. However, these battery technologies have safety and environmental concerns due to the mining of these metals and their toxicity to biological life. In addition, they are concentrated in a few countries, raising geopolitical concerns. Thus, there is now a growing demand for safer and more accessible alternatives.
I am Yeshvi, a 3rd-year Ph.D. student in Prof. Prakash’s lab at the Loker Hydrocarbon Research Institute, USC. My decision to study the battery field stems from a deep-seated passion for contributing to the global energy shift. The potential to innovate and develop a safer, affordable and more environmentally friendly alternative to existing battery technologies is incredibly motivating.
You might be familiar with AA batteries, which have positive and negative sides. Iron electrodes could serve as a negative electrode, paired with air or nickel as a positive electrode. Iron electrodes have several advantages: iron is the fourth-most-abundant metal on earth by mass, non-toxic, and can store 960 mAh of energy per gram of iron. Despite these benefits, challenges hinder the practical application of iron electrodes.
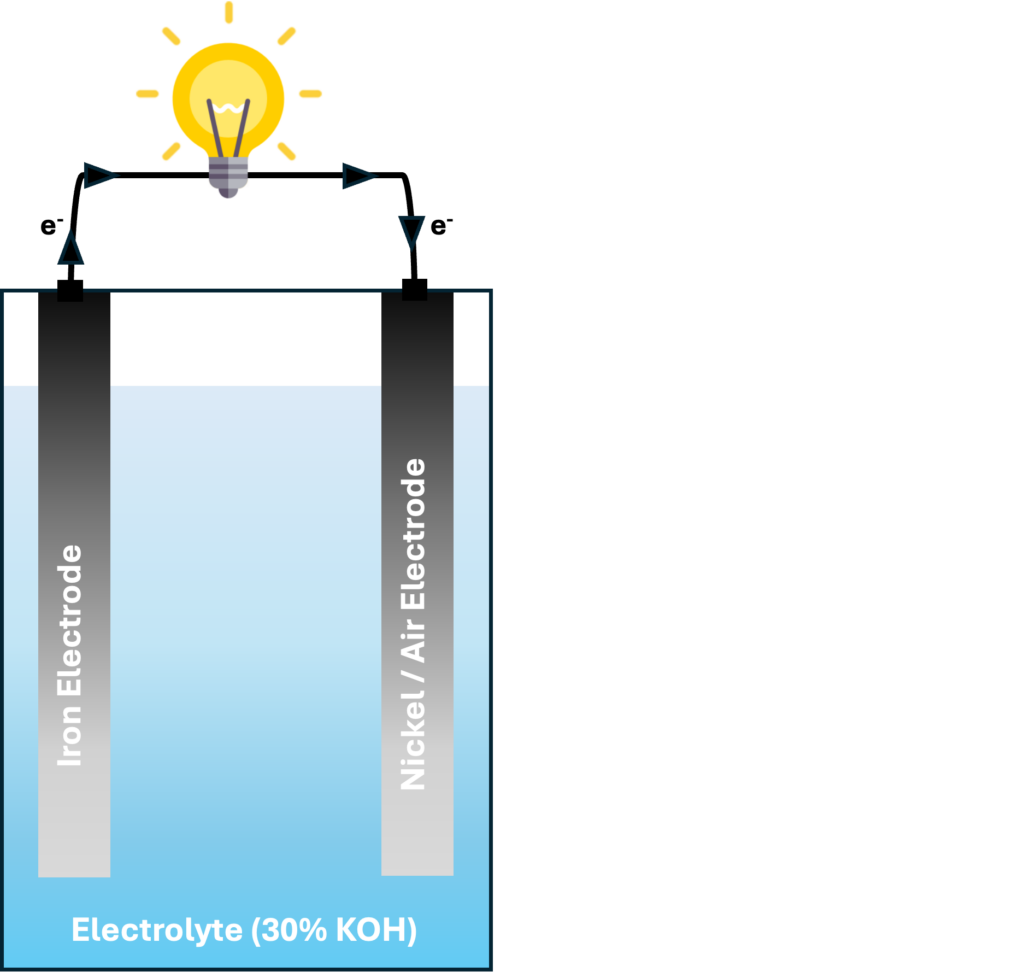
The interest in iron-air battery technology for transportation dates back to the oil crisis of the 1970s. However, by 1984, research and development were abandoned due to technical issues, such as hydrogen evolution (a normal chemical process that degrades the electrical capacity of the iron), self-discharge, water loss, difficulties protecting the iron electrode from corrosion, and oxygen reactions that reduced the efficiency of the air electrode. Recently, iron-air batteries have gained renewed interest for large-scale grid storage, requiring low-cost raw materials and long cycle life rather than high energy density. Institutions like USC, Form Energy, and the European NECOBAUT program are actively researching iron-air battery systems for automobiles and grid-level energy storage.
Supported by the Wrigley Institute Graduate Fellowship, my work in Prof. Prakash’s lab focuses on suppressing the hydrogen evolution reaction (HER) on the iron electrode. The theoretical electrical capacity of an iron electrode is as high as 962 mAhg-1, whereas in practice, less than 350 mAhg-1 is achieved. This actual attainable capacity is called the formation capacity. One of the reasons for low formation capacity is the parasitic hydrogen evolution reaction (HER), a natural chemical reaction where hydrogen forms within the iron electrode. HER results in high self-discharge rates, lower coulombic efficiency (i.e., less-efficient movement of electrons when the battery is charged or discharged), physical disintegration of the electrode, and continuous electrolyte consumption, which reduces the life of the battery.

One surprising aspect of my research has been the complexity of mitigating HER. The initial approach to suppress HER focused on modifying the electrolyte. Throughout the course of the fellowship, we investigated several additives, including sugars such as fructose, glucose, and maltose, as well as organic compounds like methanol and dimethyl sulfone. Unfortunately, these modifications did not lead to the desired improvements.
Given the limited success with electrolyte modification, our research has now shifted towards modifying the iron electrode itself. This new direction has been promising and has opened new avenues for investigation. This experience has deepened my understanding of electrochemistry and the intricacies of battery technology.
The Wrigley Institute Graduate Fellowship has been invaluable, providing not only financial support but also the opportunity to grow and communicate science effectively. The guidance and inspiration from my mentors, Prof. Narayan and Prof. Prakash, have been instrumental in shaping my approach in this challenging yet rewarding field.
My research aims to address the global challenge of efficient energy storage. By developing iron-air batteries, we hope to provide a cost-effective, sustainable solution for grid storage. This technology could alleviate geopolitical and geoeconomic issues related to the concentration of lithium and other critical metals, fostering energy storage independence especially for developing countries. Stay tuned as we continue to explore and innovate in the field of iron-air battery technology, paving the way for a cleaner and more sustainable energy future.
Yeshvi Tomar is supported by the Diane Sonosky Montgomery and Jerol Sonosky Graduate Fellowship for Environmental Sustainability Research.