
Associate Professor of Chemistry
Organic Chemistry
Ph.D., 1994, University of Wisconsin – Madison
M.S., 1987, Seoul National University
B.S., 1985, Seoul National University
Office: LHI 218
Phone: (213) 740-8768
Email: kwjung@usc.edu
Our research interests are organic synthesis, catalysis, and their applications to the fields of carbon-hydrogen bond activation, development of synthetic methodologies, and drug discovery. Current projects are as follows:
1. Design & synthesis of novel catalysts.
2. Development of synthetic methodologies using our catalysts.
3. Medicinal chemistry against rare diseases.
Design & synthesis of novel catalysts
We have designed and synthesized a number of organometallic catalysts for unprecedented and challenging reactions including carbon-hydrogen bond activation. Some of the most representative examples are novel NHC-amidate ligands and their metal complexes as shown below. In our general ligand design, we included an NHC ligand as a strong σ-donor and an amidate ligand as a strong σ-donor and possible -electron donor. Thus, our designed NHC-amidate-Pd(II) complexes have a highly electron-rich Pd center. These properties of the NHC and amidate ligands would generate stable catalysts and improve catalytic efficiency. Such enhanced reactivity may facilitate challenging catalytic reactions, i.e., C-H bond activation of hydrocarbons such as methane.
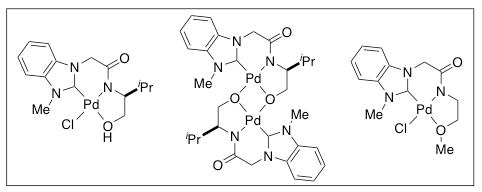
Development of synthetic methodologies using our catalysts
Our projected studies focus on the development of synthetic methodologies using our Pd catalysts, which can be either improvements over the existing methods or completely novel reactions. Some of the current projects are shown below.
Oxidative Palladium(II) Catalysis
Using oxidative Pd(II) catalysis, we have developed new synthetic methodologies, which include a modified Heck reaction. Under mild conditions, our boron-Heck reactions are highly efficient, giving rise to the stereoselective synthesis of various alkenes. As illustrated below, asymmetric catalysis has also been achieved to effect both intra- and intermolecular couplings. These methods are useful in the synthesis of medicinal compounds and natural products.
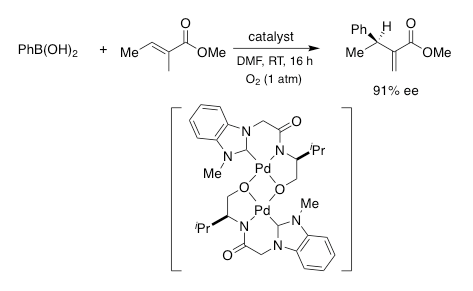
C-H Activation of Unreactive Hydrocarbons
Another approach using our newly designed NHC-amidate metal complexes is the development of novel catalytic reactions, which are challenging to carry out by utilizing known methods or catalysts. One area of our interest is the efficient C-H activation of hydrocarbons. For instance, cyclohexane was transformed to its deuterated form with 93% deuterium content and the catalytic turnover reached 536 at a mild temperature, 55 °C. Aromatic hydrocarbons such as benzene and toluene were also facile in the H/D exchange, providing up to 97% deuterium content
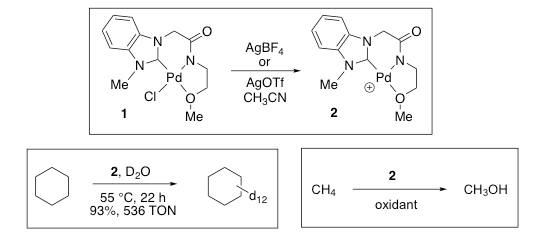
Recently, we applied similar H/D exchange conditions to gaseous hydrocarbons, particularly methane because successful results can enlighten a new way of the C-H activation and ensuing oxidation of methane. With these methods developed, we embarked on the efficient conversion of methane to liquid products such as methanol using our catalysts.
RNA Targeting Drug Discovery against Huntington’s Disease
The pathogenesis of Huntington’s disease is the expression of toxic mutant Huntingtin protein, which stems from expansions of the trinucleotide CAG-repeat tract in the HTT gene (mHTT). Wild-type and physiologically necessary Huntingtin (WT HTT) composes of up to 36 CAG repeats whereas mutant HTT (mHTT) consists of more than 40 CAG repeats. Because decreased expression of mHTT has been shown to mitigate pathology, we have sought small organic molecules to diminish the expression of mHTT to polyglutamine proteins. These compounds must selectively inhibit the expression of mHTT over WT HTT to become viable drug candidates.
We have had ideas of utilizing ex vivo assays, phenotype assays, and/or polypharmacology approaches to differentiate our program from others and to identify true viable drug candidates. By working closely with us, Prof. Peter Chung’s group has developed a novel functional assay, which is designed to evaluate the expression of both mHTT and WT HTT simultaneously. Both groups work collaboratively to refine assay methods and conduct SAR studies. We have created a novel chemical space, exhibiting selective inhibition of mHTT over WT HTT significantly.
Selected Publications
1. Oxidative Palladium(II) Catalysis: A Highly Efficient and Chemoselective Cross-Coupling Method for Carbon-Carbon Bond Formation under Base-Free and Nitrogenous-Ligand Conditions. Yoo, K. S.; Yoon, C. H.; Mishra, R. K.; Jung, Y. C.; Yi, S. W.; Jung, K. W. J. Am. Chem. Soc., 2006, 128, 16384.
2. Stereogenic Evolution of clasto-Lactacystin -Lactone from (L)-Serine. Yoon, C. H.; Flanigan, D. L.; Yoo, K. S.; Jung, K. W. Eur. J. Org. Chem., 2007, 37.
3. Asymmetric Intermolecular Heck-Type Reaction of Acyclic Alkenes via Oxidative Palladium(II) Catalysis. Yoo, K. S.; Park, C. P.; Yoon, C. H.; Sakaguchi, S.; O’Neill, J.; Jung, K. W. Org. Lett., 2007, 9, 3933.
4. Total Syntheses of ()--Kainic Acid and (+)--Allokainic Acid via Stereoselective C-H Insertion and Efficient 3,4-Stereocontrol. Jung, Y. C.; Yoon, C. H.; Turos, E.; Yoo, K. S.; Jung, K. W. J. Org. Chem. 2007, 72, 10114.
5. Chiral PdII Complexes Possessing Tridentate NHC-Amidate-Alkoxy Ligand: Access to Oxygen-Bridging Dimer Structure. Sakaguchi, S.; Yoo, K. S.; O’Neill, J.; Lee, J. H.; Stewart, T.; Jung, K. W. Angew. Chem. Int. Ed. 2008, 47, 9326.
6. Chemoselective Three-Component Coupling via A Tandem Pd Catalyzed Boron-Heck and Suzuki Reaction. O’Neill, J.; Yoo, K. S.; Jung, K. W. Tetrahedron Lett. 2008, 49, 7307.
7. Highly Regioselective Heck-Coupling Reactions of Aryl Halides and Dihydropyran (DHP) in the Presence of NHC-Pyridine Ligand. Jarusiewicz, J.; Yoo, K. S.; Jung, K. W. Synlett, 2009, 482.
8. Air/Water-Stable Tridentate NHC-PdII Complex; Catalytic C-H Activation of Hydrocarbons via H/D Exchange Process in D2O. Lee, J. H.; Yoo, K. S.; Park, C. P.; Olsen, J. M.; Sakaguchi, S.; Prakash, G. K. S.; Mathew, T.; Jung, K. W. Adv. Synth. Cat. 2009, 351, 563.
9. Efficient Three-Component Strecker Reaction of Aldehydes/Ketones via NHC-Amidate Palladium(II) Complex Catalysis. Jarusiewicz, J.; Choe, Y.; Yoo, K. S.; Park, C. P.; Jung, K. W. J. Org. Chem., 2009, 74, 2873.
10. Expeditious Enyne Construction from Alkynes via Oxidative Pd(II) Catalyzed Heck-Type Coupling. Hadi, V.; Yoo, K. S.; Jeong, M.; Jung, K. W. Tetrahedron Lett. 2009, 50, 2370.
11. Formal Aromatic C-H Insertion for Stereoselective Isoquinolinone Synthesis and Studies on Mechanistic Insights into the C-C Bond Formation. Park, C. P.; Nagle, A. S.; Yoon, C. H.; Chen, C.; Jung, K. W. J. Org. Chem. 2009, 74, 6231.
12. Tridentate, anionic tethered N-heterocyclic carbene of Pd(II) complexes. Sakaguchi, S.; Kawakami, M.; O’Neill, J.; Yoo, K. S.; Jung, K. W. J. Organometal. Chem. 2010, 695, 195.
13. Asymmetric Intermolecular Boron-Heck Type Reactions via Oxidative Palladium(II) Catalysis with Chiral Tridentate NHC-Amidate-Alkoxide Ligands. Yoo, K. S.; O’Neill, J.; Sakaguchi, S.; Giles, R.; Lee, J. H.; Jung, K. W. J. Org. Chem. 2010, 75, 95.
14. Efficient Diacetoxylation of Alkenes via Pd(II)/Pd(IV) Process with Peracetic Acid and Acetic Anhydride. Park, C. P.; Lee, J. H.; Yoo, K. S.; Jung, K. W. Org. Lett. 2010, 12, 2450.
15. Chemoselective hydroamination of vinyl arenes catalyzed by an NHC-amidate-alkoxide Pd(II) complex and p-TsOH. Giles, R.; O’Neill, J.; Lee, J. H.; Chiu, M. L.; Jung, K. W. Tetrahedron Lett. 2013, 54, 4083.
16. Dual studies on a hydrogen-deuterium exchange of resorcinol and the subsequent kinetic isotope effect. Giles, R.; Kim, I.; Chao, W. E.; Moore, J.; Jung, K. W. J. Chem. Educ., 2014, 91, 1220.
17. Hydrogen–deuterium exchange of aromatic amines and amides using deuterated trifluoroacetic acid. Giles, R.; Lee, A.; Jung, E.; Kang, A.; Jung, K. W. Tetrahedron Lett. 2015, 56, 747.
18. H-D exchange in deuterated trifluoroacetic acid via ligand-directed NHC-palladium catalysis: a powerful method for deuteration of aromatic ketones, amides, and amino acids. Giles, R.; Ahn, G.; Jung, K. W. Tetrahedron Lett. 2015, 56, 6231.
19. Hydroalkenylation: Palladium catalyzed co-dimerization of unactivated alkenes. Zargari, N.; de Prevoisin, G.; Kim, Y.; Kaneshiro, K.; Runberg, R.; Park, J.; LaCroix, K.; Narain, R.; Lee, B. D.; Lee, J. H.; Jung, K. W. Tetrahedron Lett., 2016, 57, 815.
20. Unexpected, Latent Radical Reaction of Methane Propagated by Trifluoromethyl Radicals. Zargari, N.; Winter, P.; Liang, Y.; Lee, J. H.; Cooksy, A.; Houk, K.; Jung, K. W. J. Org. Chem., 2016, 81, 9820.
21. Carbon dioxide hydrogenation: Efficient catalysis by an NHC-Amidate Pd(II) complex. Zargari, N.; Jung, E.; Lee, J. H.; Jung, K. W. Tetrahedron Lett., 2017, 58, 3330.
22. Nitrohydroxylation of Olefins with Nitric Acid Using Tridentate NHC–amidate–alkoxide Containing Palladium Catalysts. O’Neill, J.; Lee, J. H.; Kim, S.; Zargari, N.; Ketabchi, B.; Akahoshi, J.; Yang, F.; Jung, K. W. Topics in Catal., 2018, 61, 630.
23. Compositions and Methods for Treating or Ameliorating Coronavirus Infections. Jung, K. W.; Comai, L. US Patents, 2021, US 63/194,449.
24. Methane Sulfonation via a Free Radical Mechanism by Trifluoroacetylsulfuric Acid. Kim, S.; Wang, J.-C.; Lee, J. H.; Jung, K. W. J. Org. Chem., 2022, 87, 10539.
25. Novel Methane Activation by Sulfur Dioxide and Molecular Oxygen via Trifluoroacetylsulfuric acid. Kim, S.; Wang, J.-C.; Lee, J. H.; Jung, K. W. Green Chem., 2022, 24, 7918.
26. Hydrogen-Deuterium Isotope Exchange of Methane via Non-redox Palladium Catalysis. Kim, S.; Lee, J. H.; Jarusiewicz, J.; Wang, J.-C.; Jung, K. W. Eur. J. Inorg. Chem., 2023, 26, e202200615.
27. Efficient Conversion of Methane to Methanesulfonic Acid via Trifluoroacetylsulfuric Acid. Jung, K. W.; Kim, S.; Wang, J.-C.; Lee, J. H. US Patents, 2023, US 2023/0242477 A1.
28. Mechanistic Investigations on the Direct Regioselective Sulfonation of Gaseous Light Hydrocarbons by Sulfur Dioxide. Wang, J.-C.; Kim, S.; Lee, J. H.; Ascencio, C.; Chung, L.; Cashman, J.; Jung, K. W. ChemCatChem, 2025, 17, e202200615.
29. Aromatic sulfonation with sulfur dioxide via Trifluoroacetylsulfuric acid. Wang, J.-C.; Kim, S.; Lee, J. H.; Ascencio, C.; Jung, K. W. Tetrahedron Lett., 2025, 167, 155677.