Image from Fig. 1 of “Development and characterization of amino donor-acceptor Stenhouse adducts” published in Nature Communications
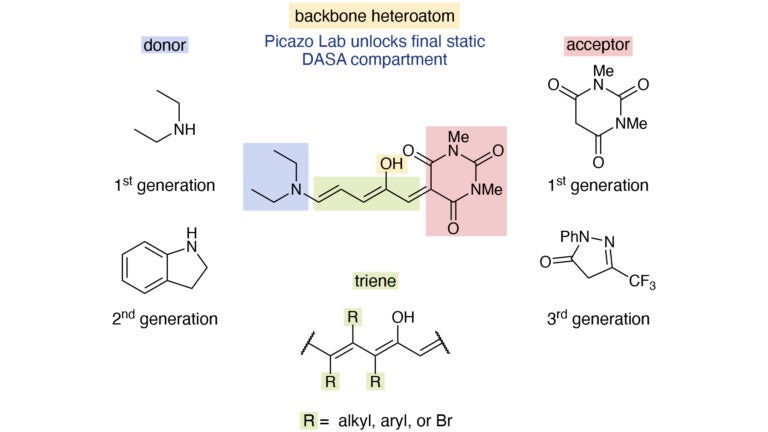
Members of the Picazo Group have published a paper entitled “Development and characterization of amino donor-acceptor Stenhouse adducts” in Nature Communications that explains their recent discovery. The paper describes the strategy and methodology for a novel rearrangement that addresses chemical limitations first encountered in the 1850s, and its application to create new chemical matter that reversibly responds to light named donor-acceptor Stenhouse adducts (DASAs). This breakthrough in reactivity unlocks the backbone heteroatom, the final structural compartment in DASAs that had previously eluded laboratory research. Principal Investigator Elias Picazo has thereby expanded his expertise with respect to addressing age-old limitations in organic synthesis and small molecule material design.

In 1850, a type of heterocycle called furfural was used by chemist John Stenhouse to make Stenhouse salts. Since the 1970s, Giovanni Piancatelli and others have been developing furan ring rearrangements using furyl carbinols to make important organic molecules like natural products and pharmaceutical scaffolds. Peter Šafář and his team discovered that activated furans can be used to make DASAs in 2000; and Javier Read de Alaniz and colleagues discovered that DASAs were reversibly responsive to light in 2014. From the time of this discovery to present-day, seminal studies determined that minute structural modifications result in significant changes in the photophysical properties of DASAs. While three of four molecular compartments had been rendered synthetically modular, all previous attempts by organic chemists to unlock the fourth compartment were unsuccessful.
In developing a strategy to increase applications of DASAs, Picazo observed that rearrangement chemistry required to make the photoswitches was previously limited to furans. Furan rearrangements have found broad use in material, natural, and pharmaceutical product synthesis because they possess the lowest degree of aromatic stabilization when compared to pyrrole and thiophene heterocycles. His lab therefore experimented with pyrrole starting materials and leveraged the new rearrangement chemistry to finally unlock the fourth compartment of these photoswitchable molecules, solving a mystery that had loomed over the field of organic chemistry for nearly a decade.
Once the backbone heteroatom was unlocked, the Picazo Group was able to perform a simple atomic substitution of oxygen with nitrogen to create a new class of DASAs. Given that nitrogen can form more covalent bonds, a fifth compartment of the molecule was created. Further investigation showed that the nitrogen substituent impacts the molecule’s photoswitchable behavior, making it less responsive to light. This behavior is described in the paper and will inform future projects in Picazo’s lab. The group speculates that DASAs can be used on polymers or biomolecules in light- or chemo-sensing applications, though this is still unproven due to the early stage of the research. A forthcoming article will expand on this multifaceted discovery, focusing on how the fifth compartment can be used to develop more functional and efficient photoswitches. The more control chemists have with synthetic modulation of DASAs, the more potential applications they will have in related scientific fields.