The Bradforth Group expands the use of Liquid Jet Photoelectron Spectroscopy
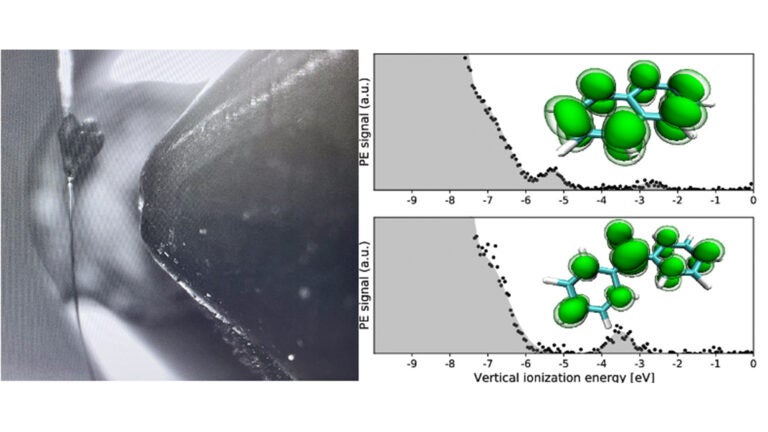
An emerging spectroscopic methodology, liquid jet photoemission has only been applied previously to water and low volatility solvents. While liquid jet photoelectron spectroscopy (LJ-PES) probes just ten nanometers or less below the surface of a liquid jet, the technique still provides a great deal of information about the solutes at the vacuum-liquid interface as well as the bulk of a given solution. Its potential to examine intermediates in synthetic mechanisms therefore demands learning how to use it for a wider range of solvents. The Bradforth Group has been the first to use this technique with the solvent tetrahydrofuran (THF) in vacuo. The group set out to investigate the electronic structure of aromatic radical anions in the solution phase. They were then able to gain a greater understanding of the stabilization of these anions through analysis of LJ-PES measurements. The group and their collaborators have described their research in a paper published in the March 2024 issue of the Journal of the American Chemical Society and were featured on the JACS cover. Their findings are relevant to the Birch reduction process, a fundamental reaction in organic chemistry that is used to convert arenes to cyclodienes, as they contribute to optimizing conditions for an industrial process used to create products such as pharmaceutical drugs and perfumes. Ethereal and ammoniacal solvents (such as THF and ammonia, respectively) are the only solvents known to support Birch reduction chemistry. The group has previously succeeded in carrying out LJ-PES in liquid ammonia.
At the tail end of the COVID-19 pandemic, Prof. Stephen Bradforth traveled to Berlin to perform the first round of measurements at the BESSY II electron storage ring at Helmholtz-Zentrum Berlin (HZB) für Materialien und Energie. The next phase of experimentation was done by Brandon Young, a PhD student in Chemistry at USC, and was conducted at the Fritz-Haber Institute (FHI) of the Max-Planck Society to extend the measurements and demonstrate reproducibility on a different instrument. The experimental team included senior researchers Christian Schewe and Pavel Jungwirth of the Institute of Organic Chemistry and Biochemistry-Prague (IOCB-Prague), former USC postdoc and HZB-Berlin staff scientist Robert Seidel, and longtime group collaborator Bernd Winter of FHI. According to Bradforth, “The advantage of photoelectron spectroscopy is that the measurements directly read out the frontier orbital energies for two reaction intermediate radical anions within the environment used for synthetic chemistry.” Quantum chemical calculations from IOCB-Prague doctoral students Tatiana Nemirovich and Krystof Brezina were used to assign the data and understand where the excess charge lies in the naphthalene and benzophenone radical anions. Through comparison of relative vacuum ionization energies with values from cyclic voltammetry (CV), the researchers could quantify the chemical stability of the two radical anions. An important advance in the work was in LJ-PES calibration by introducing a bias to the liquid jet so that the precision of the reduction potentials found through LJ-PES are now comparable to the electrochemistry “gold standard” of CV.
Brandon Young explains that the study raises interesting questions about breaking aromaticity. One experiment included in this research allowed him and his colleagues to “press pause” before a proton is added during the conversion of naphthalene to its hydrogenated form in liquid THF. In doing so, they were able gain more insight into the stabilization process of the radical anion intermediate, which is the first step of this conversion. “The question becomes: why would the neutral aromatic naphthalene go from being a very happy and stable aromatic ring to accepting an electron and breaking its aromaticity in the first place?” They concluded that the Birch reaction is optimized by the electrostatic environment provided by the solvent along with the positioning of the counter cation occuring in either an amine-containing solvent or an ethereal one. “The better we understand the Birch reaction in these larger aromatics, the closer we get to studying the simplest aromatic anion, benzene,” Young added. In the meantime, their lab will continue working towards the goals of observing a textbook example of the benzene radical anion by stabilizing it in the liquid phase and applying LJ-PES to more solvents that support chemical synthesis.