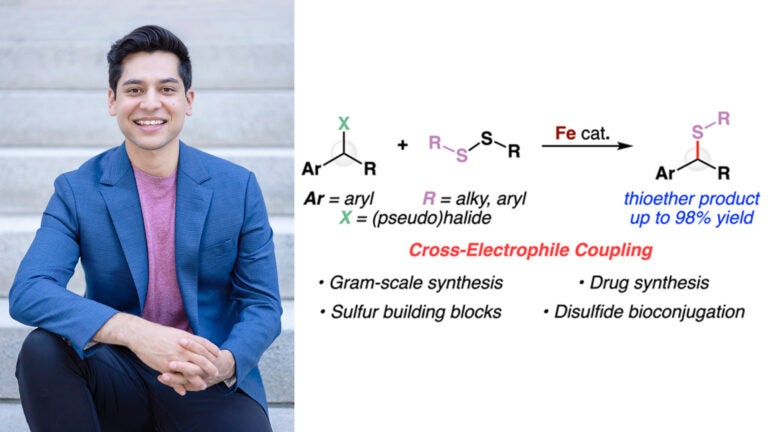
Prof. Elias Picazo and the newly discovered iron-catalyzed reaction (image published in the Journal of the American Chemical Society)
The element of iron is widely abundant in nature and crucial for a range of biochemical processes such as oxygen transport and DNA synthesis. While it is known that iron is essential to most living organisms and has low toxicity, there are new platforms of reactivity that have yet to be discovered in the field of iron catalysis. The Picazo lab has contributed to a significant advance in this field which is detailed in an article of the February 2024 issue of the Journal of the American Chemical Society. Principal Investigator, Elias Picazo, discovered that iron is an effective catalyst for coupling benzyl halides with disulfides without adding an external reductant and in the absence of photoredox conditions. The lab then ran several experiments which confirmed the reaction is effective for a variety of starting materials, providing solid evidence that it is a general approach to make molecules with carbon–sulfur bonds. The discovery of this new reactivity is encouraging with respect to sustainability insofar as ample amounts of iron can be found in Earth’s crust and using this particular transition metal in lieu of other substances could help to avoid releasing harmful toxins into the environment.
An electrophile is an atom or molecule that seeks to bond with an atom or molecule that contains an electron pair. While it is more common for an electrophile to couple with a nucleophile, it is possible to couple two electrophiles together. What makes Picazo’s discovery unique is that it is the first reaction to couple benzyl halides with disulfides (two electrophiles) without an external reductant and photoredox conditions. Experimentation later revealed that bromide on the starting material acts as an internal reductant.
Initially, the lab applied their concept to yield thioether products with potential uses in the fields of medicine, agriculture, and more. Once it was concluded that it is general in its scope, the lab successfully carried the reaction out on gram-scale—a harbinger for industrial application. The thioether products were then subjected to another reaction to change their forms (i.e., derivatized) to sulfoxide and sulfone, which are building blocks for several transformations that have been established in the field of chemistry. Disulfides are commonly found in biomolecules such as peptides, so the Picazo lab has theorized that disulfide bioconjugation performed via this reaction may be used to tag biomolecules with fluorophores, molecules that interact with light to image track movement in the body of a living organism. Such tracking experiments could provide new insights about the given biomolecule’s function.
It was noted in the publication that this iron-catalyzed coupling is currently limited to benzylic (pseudo)-halide substrates and that the reason for this is still unknown. Along with future projects extending this work, the Picazo lab intends to investigate the limitation. Other experiments involved in this discovery, including a spin trap experiment, seem to suggest that the reaction is going through a radical mechanism, or a single electron process. While the evidence is strong, the Picazo lab aims to test this hypothesis further.
As previously explained, the reaction can be applied through the development of highly useful thioether products which can further serve innovations in the field of chemistry and/or be scaled up to meet the needs of industrial production. “While this is certainly promising for industry, as a chemist, I’m most excited about the discovery of new reactivity,” stated Prof. Picazo. He went on to say, “I’m also excited to see what discoveries this new platform of catalysis will bring.” This work was supported by funding through an NIH/NIGMS R00 grant and made possible through resources such as the NMR Core Facility at the USC Center for Excellence for Molecular Characterization and laboratory space in the Loker Hydrocarbon Research Institute at USC. The Picazo lab will continue to research this iron-catalyzed reaction, its potential applications, and the mechanisms involved that remain to be fully understood.