The Pratt Lab Helps to Advance Treatment of Neurodegenerative Diseases
The Pratt lab has recently made an exciting discovery regarding posttranslational modifications (PTMs) which is described in their publication in the February 2024 issue of Nature Chemical Biology. To make this discovery, the lab used a combination of techniques from the fields of organic synthesis, genetics, protein biochemistry, and cellular biology, including protein characterization by mass spectrometry at the Agilent Center of Excellence in Biomolecular Characterization. The lab was one of the first to exploit the method of protein synthesis for the site-specific installation of O-GlcNAc, which is the only one that allows scientists to carry out site-specific and homogenous installation of this particular PTM. This builds on prior research from the Pratt lab and others, establishing greater understanding of how to approach the treatment of elusive, neurodegenerative diseases (NDDs), such as Alzheimer’s, Parkinson’s, and Multiple Systems Atrophy.
Previous studies on patients afflicted with Parkinson’s disease have shown that the protein a-synuclein (a-Syn) plays a main role in the spreading of NDDs. When this protein aggregates and forms amyloid fibrils, the overall process leads to neuron death and appears to spread from one area of the brain to another by seeding activity. In order to prevent and treat NDDs, scientists are exploring ways to inhibit fibril formation as well as the seeding activities and toxicities of these fibrils. It is particularly challenging to develop general therapeutic strategies with respect to a-Syn as this protein can produce a variety of fibril structures or strains, toxicities, and seeding capabilities. This is precisely why the Pratt lab aims to meet this challenge head on by focusing much of its research on the pathogenic properties of a-Syn.
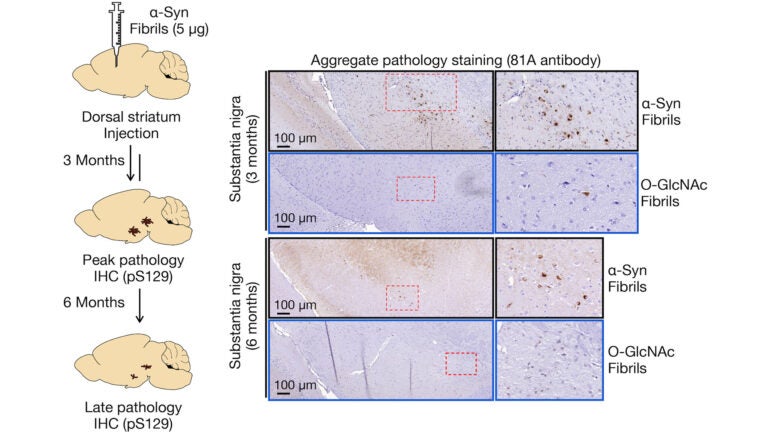
After a protein is translated, various PTMs can occur, affecting the structure of the proteins as well as their biochemical interactions. In fact, it is now widely understood that the human proteome is far more complex than the human genome, partly due to protein PTMs. Glycosylation, a type of PTM, involves the attachment of a carbohydrate to a macromolecule (typically a protein or lipid). Given that the intracellular glycosylation O-GlcNAc has been found on multiple sites of a-Syn, it is of great interest to protein biochemists and the scientific community at large. Through previous experimentation, the Pratt lab has shown that O-GlcNAc reduces the kinetics of a-Syn fibril formation with site-dependent differences. They have extended this work to determine if O-GlcNAc specifically at the site of serine 87 impacts the protein’s strain formation, seeding capabilities, toxicity, and pathogenicity.
What the lab’s experimentation revealed is that O-GlcNAc modified amyloids induced far less pathology in neurons and in the brains of mice when compared to unmodified a-Syn fibrils. Using cryoEM, they further determined that the modified fibrils differed in structure from the unmodified fibers as well as amyloids characterized from Parkinson’s disease and Multiple Systems Atrophy. These findings have several implications. By inhibiting O-GlcNAcase, an enzyme that removes O-GlcNAc, O-GlcNAc may then be elevated in order to slow the progression of various NDDs. Compounds to inhibit the enzyme have been implemented in Phase II studies. The Pratt lab researchers and others have contributed solid evidence that this modification would be an effective means for novel therapeutic drugs to not only decrease pathogenic amyloid strains but also slow the potentially harmful aggregation of certain proteins. It should be noted, however, that the lab’s findings also suggest that not all fibrils in the brain are pathogenic; and so, future research on the correlation between pathology formation and the progression of NDDs or drug efficacy must account for non-pathogenic fibrils.
The Pratt lab worked in collaboration with Prof. Hilal Lashuel’s lab at EPFL (École polytechnique fédérale de Lausanne) and with research contributions from University of Pennsylvania and UT Southwestern Medical Center to make its recent discovery. Regarding future implications, Principal Investigator Matthew Pratt remarked, “As more inhibitors that increase O-GlcNAc enter the clinic, it is important to understand their consequences on critical proteins in neurodegenerative diseases, and our approach is a few that can directly address these open questions.” In addition to opening new scientific inquiries relating to a-synuclein, O-GlcNAc, and PTMs, it will undoubtedly serve to advance the development of medical interventions for a range of neurodegenerative diseases.