Researchers may have found one path to a longer life
Scientists at USC Dornsife College of Letters, Arts and Sciences may have found the beginnings of a path toward increasing human lifespan.
The research, published July 10 in the Journal of Gerontology: Biological Sciences, shows the drug mifepristone can extend the lives of two very different species used in laboratory studies, suggesting the findings may apply to other species, including human beings.
Countering wear and tear inflicted by males
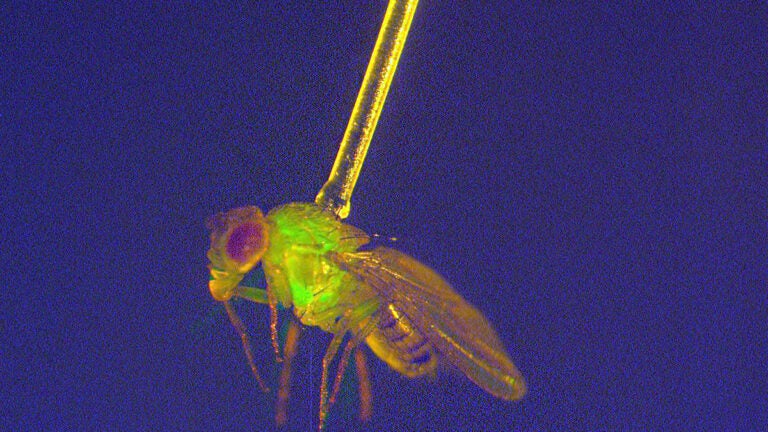
Studying one of the most common laboratory models used in genetic research — the fruit fly Drosophila — John Tower, professor of biological sciences, and his team found that the drug mifepristone extends the lives of female flies that have mated.
Mifepristone, also known as RU-486, is used by clinicians to end early pregnancies as well as to treat cancer and Cushing disease.
During mating, female fruit flies receive a molecule called sex peptide from the male. Previous research has shown that sex peptide causes inflammation and reduces the health and lifespan of female flies.
Tower and his team, including Senior Research Associate Gary Landis, lead researcher on the study, found that feeding mifepristone to the fruit flies that have mated blocks the effects of sex peptide, reducing inflammation and keeping the female flies healthier, leading to longer lifespans than their counterparts who did not receive the drug.
The drug’s effects in Drosophila appear similar to those seen in women who take it.
“In the fly, mifepristone decreases reproduction, alters innate immune response and increases life span,” Tower explained. “In the human, we know that mifepristone decreases reproduction and alters innate immune response, so might it also increase life span?”
Overcoming juvenile hormone effects

John Tower’s research focuses on the molecular biology of aging. (Photo: Gus Ruelas.)
Seeking a better understanding of how mifepristone works to increase lifespan, Tower and his team looked at the genes, molecules and metabolic processes that changed when flies consumed the drug. They found that a molecule called juvenile hormone plays a central role.
Juvenile hormone regulates the development of fruit flies throughout their life, from egg to larvae to adult.
Sex peptide appears to escalate the effects of juvenile hormone, shifting the mated flies’ metabolism from healthier processes to metabolic pathways that require more energy to maintain. Further, the metabolic shift promotes harmful inflammation, and it appears to make the flies more sensitive to toxic molecules produced by bacteria in their microbiome.
Mifepristone changes all of that.
When the mated flies ate the drug, their metabolism stuck with the healthier pathways, and they lived longer than their mated sisters who did not get mifepristone. Notably, these metabolic pathways are conserved in humans, and are associated with health and longevity, said Tower.
Hope for humans?
In a scientific first, Tower and collaborators Chia-An Yen, who obtained her Ph.D. last spring from USC Dornsife, and Sean Curran, associate professor of gerontology and biological sciences at USC Leonard Davis School of Gerontology and USC Dornsife, also gave mifepristone to another common laboratory model, a small roundworm called C. elegans. They found the drug had the same life-extending effect on the mated worm.
Because Drosophila fruit flies and C. elegans worms sit on relatively distant branches of the evolutionary tree, Tower believes the similar results in such different species suggest other organisms, including humans, might see comparable benefits to lifespan.
“In terms of evolution, Drosophila and C. elegans are equally as distant from each other as either one is distant from humans,” he said, and the fact that mifepristone can increase lifespan in both species suggests the mechanism is important to many species.
Tower emphasizes that a clearer understanding of the intricacies of mifepristone’s actions is needed before drawing any firm conclusions.
“Our data show that in Drosophila, mifepristone either directly or indirectly counteracts juvenile hormone signaling, but the exact target of mifepristone remains elusive.”
Revealing that target may give scientists critical insight needed to extend lifespan in people.
About the study
In addition to Tower, Landis, Yen and Curran, authors on the study include Devon Doherty, Yang Fan, Ina Wang, Jimmy Wu, Palak Patel, Shinwoo Lee, and Mina Abdelmesieh at USC Dornsife; Lu Wang and Daniel Promislow of the University of Washington, Seattle; and Jie Shen of Hangzhou Dianzi University in China.
The study was supported by National Institute on Aging at the National Institutes of Health (NIH) grant R01 AG057741 and NIH grants P30 AG013280, R01 AG049494 and R01 AG063371.