T.1(1) – Cryophorus
This demonstrates freezing by evaporation. The glass tube has a bulb on each end partly filled with water at reduced pressure so that the vapor content of the tube is high. When one bulb is placed in a mixture of dry ice and alcohol, water in the upper bulb evaporates so quickly that it freezes. Light is projected through the tube to show the ice crystals forming. The tubes overall length is 33 cm.
T.1(2) – Metal Can
It is a light metal can, about 20 cm x 10 cm x 25 cm, with a threaded lid. A little water is poured in the can and it is heated over a flame. As the water boils and the can is filled with steam, set it aside and close the lid, sealing it tightly. After a while, as the steam begins to condense, the atmospheric pressure crushes the can. The same principle can be applied using a soda can; by boiling water in an emptied soda can and immediately turning it upside down in a cold water-filled container, the can will crumple and collapse.
Click here to see a video clip of the can collapse demo.
T.1(3a) – Liquid Nitrogen Demonstration
Many objects may be used to demonstrate the law of internal heat as they have their temperatures lowered to the liquid Nitrogen’s temperature.
- Rubber will become inelastic: a rubber ball will not bounce after being dipped in liquid Nitrogen and rubber hose can be crushed like glass with a hammer.
- A flower will also break like glass after being dipped in liquid Nitrogen
- Small balloons filled with air will be completely deflated while in liquid Nitrogen and re-inflate themselves as they are allowed to warm up to room temperature. Helium-filled balloons will deflate and drop to the floor, rising to the ceiling after warming up
- A small, high-temperature superconductor will float a smaller magnet when cooled to liquid nitrogen temperature, demonstrating the Meissner Effect
- A balloon is filled with gas Oxygen and attached to a test tube. The test tube is dipped in liquid Nitrogen. After a few minutes, there will be a small amount of bluish liquid on the tube’s bottom: liquid Oxygen (90 K or -183°C)
24 Hour notice required for setup since we get the liquid Nitrogen at SSC
T.1(3b) – Ping Pong Ball Liquid Nitrogen Demo
For this demo approximately 100 colored ping pong balls placed in a white pail. For safety reasons, the pail is then placed in a shatterproof plexiglass container. Small quantity of liquid nitrogen is poured into a plastic water bottle and then sealed. Then the pressure of cold, trapped nitrogen is placed in the bucket with the ping pong balls. After a short period of time, the liquefied gas expands causing the water bottle to burst and the ping pong balls hurled into the air.
NOTE: 48 Hour notice required for setup


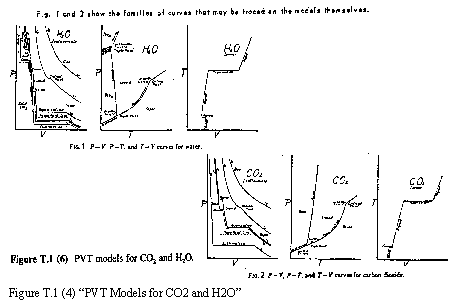
T.1(4) – PVT Models for CO2 and H2O
These models show the thermodynamic surfaces for CO2 and H2O, representing the P-V-T relations for all phases: gas, vapor, liquid, and solid. The exaggerated scale of the transitions makes easier the visualization of the volume changes as will as all the behavior of CO2 and H2O throughout their change of state. Each model is molded in a 25 cm side cube, with highly visible colors.
T.1(5) – Pulse Gas
For demonstrating the increase in vapor pressure with temperature. Two connecting glass bulbs are partly filled with a volatile, tinted red liquid and with the air partially evacuated. Heat from one’s hand is sufficient to cause rapid transfer of liquid into the other bulb, where it bubbles rapidly.
T.1(6) – Super-Cooled Demo
It demonstrates crystallization and the subsequent release of latent heat. The super-cooled and super-saturated pack (15 cm x 7.5 cm) contains a solution made of sodium acetate and water, and a beryllium-copper disk (1.5 cm). Flexing the disk triggers two simultaneous and rapid chain reactions. Stored energy is released throughout the solution as it crystallizes. In about five seconds, the pack can reach a temperature up to 40°C (130°F) and will stay warm for about two hours. It is reusable and there are three samples in the lab.
