Cell-Based Therapeutics
Background
Cell-based therapeutics involve the use of genetically engineered immune cells to target and destroy cancer cells. Two major types of these therapies are Chimeric Antigen Receptor T-cell (CAR-T) therapy and T-cell Receptor (TCR) therapy. Both CAR-T and TCR therapies represent cutting-edge advancements in cell-based cancer treatments, offering new hope for patients with certain types of cancer. CAR-T therapy has shown remarkable success in treating hematologic malignancies, while TCR therapy holds promise for targeting a broader range of cancers, including solid tumors. Each approach has its own set of advantages and challenges, and ongoing research aims to optimize these therapies for better efficacy, safety, and broader applicability.
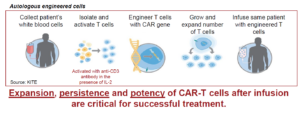
CAR-T cells have been approved for use in the treatment of hematologic malignancies. Theranostic approaches are required to help predict efficacy and monitor response in individual patients. Current strategies used to identify the presence of CAR-T cells in blood or bone marrow rely on quantitative polymerase chain reaction (qPCR) and minimalistic flow cytometry antibody panels. Due to limiting cell numbers at time points beyond 1-month post-infusion, little is known about whether the persisting gene marked CAR-T cells are functional in terms of surface CAR expression, phenotype, proliferative index, and other aspects of immune function such as expression of exhaustion markers that may associate with durable response or disease relapse. The CAR-T field’s most current understanding of biomarkers of clinical efficacy reflects a general observation that the magnitude of CAR-T expansion in peripheral blood, within 2 weeks post-treatment, correlates with objective response and toxicity. A central unanswered question is functional relevance of long-term persisting CAR-T cells in patients with ongoing complete response or durable partial response.
Patient-derived TCRs are tailored to the specific antigens presented by a patient’s tumor, providing a highly personalized treatment approach. Can target a broader range of cancers, including solid tumors, by recognizing intracellular and surface antigens presented by MHC. This enhances the ability of T cells to recognize and kill cancer cells with greater precision.
This program is focused on the development of 1) a rare cell identification assay to detect and analyze cellular therapy and 2) monitor the landscape of tumor cells in the peripheral blood.
Current Studies
- Metastatic Prostate Cancer
- Prostate stem cell antigen (PSCA) targeted CAR-T
- Prostate-specific membrane antigen (PSMA) targeted CAR-T
- Metastatic Breast Cancer
- Human epidermal growth factor receptor 2 (HER2) targeted CAR-T
- Metastatic Ovarian Cancer
- Tumor-associated glycoprotein 72 (TAG-72) targeted CAR-T
- Metastatic Pancreatic Cancer
- mutant KRAS (mKRAS) targeted TCR
News and Publications
- Shishido S, Hart O, Jeong S, Moriarty A, Heeke D, Rossi J, Bot A, Kuhn P. Liquid biopsy approach to monitor the efficacy and response to CAR-T cell therapy. Journal of ImmunoTherapy of Cancer. 2024;12:e007329. doi:10.1136/jitc-2023-007329.
-
Dorff T, Blanchard MS, Adkins LN, Luebbert L, Leggett N, Shishido SN,…D’Apuzzo M, Kuhn P, Pachter L, Forman S, Priceman S. PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-02979-8
- CancerNetwork.com: Assessing the Benefit of CAR T-cell Therapies for mCRPC (cancernetwork.com)
Contact Us
USC Michelson Center
Convergent Science Institute in Cancer
1002 Childs Way
Los Angeles, CA 90089