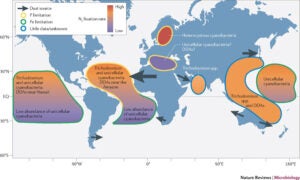
Relative N2 fixation and information on the prevailing limiting nutrient are shown in each area by the fill colour and outline, respectively, and the source and relative strength of dust sources are shown by the size of the grey arrows. The diazotrophic species that dominate in each basin are indicated, with other types noted if they are found at high abundance in specific environments. Much of the abundance data available come from late spring–early autumn in each hemisphere, when higher overall abundances are expected. Robust seasonal data sets of diazotroph
biomass are only available for limited locations, so it is
less clear how the global patterns may change
temporally. DDA, diatom diazotroph association; EQ, equator.
Relative N2 fixation and information on the prevailing limiting nutrient are shown in each area by the fill colour and outline, respectively, and the source and relative strength of dust sources are shown by the size of the grey arrows. The diazotrophic species that dominate in each basin are indicated, with other types noted if they are found at high abundance in specific environments. Much of the abundance data available come from late spring–early autumn in each hemisphere, when higher overall abundances are expected. Robust seasonal data sets of diazotroph biomass are only available for limited locations, so it is less clear how the global patterns may change temporally. DDA, diatom diazotroph association; EQ, equator.
Key Points:
- N2 fixation in the ocean is an important process that contributes to the biological sequestration of CO2 in the deep ocean.
- There are different types of marine diazotrophs (including heterotrophic bacteria), but those that are known to contribute to marine N2 fixation are: members of the genus Trichodesmium, which are non-heterocystous, filamentous cyanobacteria; the unicellular cyanobacteria UCYN-A and Crocosphaera watsonii; free-living heterocystous species such as Nodularia spp. and Anabaena spp.; and the heterocystous diatom symbiont Richelia intracellularis. Although all of these are cyanobacteria, it is predicted that N2 fixation in UCYN-A is dependent on organic C (that is, UCYN-A is a secondary producer), whereas the others are primary producers.
- The temperature defines the boundaries of where each type of organism can be found, and the deposition of dust (and in the case of the Baltic Sea the runoff from land), the nutrient supply and the internal cycling of nutrients control which nutrients (Fe or P) will be limiting to diazotrophs. Diazotrophs have developed a number of physiological adaptations to deal with both types of nutrient limitation.
- Fe and P also seem to control the distribution of diazotrophic species in the major ocean basins, with the high Fe areas of the North Atlantic Ocean and Arabian Sea hosting high abundances of Trichodesmium spp., whereas the North and South Pacific Ocean and South Atlantic Ocean seem to be dominated by unicellular cyanobacterial diazotrophs.
- The distribution of diazotrophic species is important, and different morphological and physiological types have the potential to affect the mode of transfer of newly fixed N into the food web and the sequestration of atmospheric CO2 in the deep sea.
- Research is now focusing on how increased atmospheric CO2 affects N2 fixation, and on the search for N2 fixation in waters where it has not been previously considered: that is, lower-temperature waters and waters with measureable amounts of fixed inorganic N. High CO2 can increase N2 fixation in Trichodesmium spp. and C. watsonii.
Reference:
Emerging patterns of marine nitrogen fixation
Nature Reviews Microbiology 9, 499-508 (July 2011) |
doi:10.1038/nrmicro2594
Jill A. Sohm, Eric A. Webb & Douglas G. Capone