Converging Science and Engineering
A new microscopy technology allows scientists to view single molecules in living animals at higher-than-ever resolution, and in a trial run has revealed new findings on the causes of muscular dystrophy.
Dubbed “Complementation Activated Light Microscopy” (CALM), the new technology allows imaging resolutions that are an order of magnitude finer than conventional optical microscopy, providing new insights into the behavior of biomolecules at the nanometer scale.
In a paper published on Sept. 18 by Nature Communications, the researchers behind CALM used it to study dystrophin – a key structural protein of muscle cells – in Caenorhabditis elegans worms used to model Duchenne muscular dystrophy.
Duchenne muscular dystrophy is the most severe and most common form of the degenerative disease.
The researchers showed that dystrophin was responsible for regulating tiny molecular fluctuations in calcium channels while muscles are in use. The discovery suggests that a lack of functional dystrophin alters the dynamics of ion channels — helping to cause the defective mechanical responses and the calcium imbalance that impair normal muscle activity in patients with muscular dystrophy.
CALM works by splitting a green fluorescent protein from a jellyfish into two fragments that fit together like puzzle pieces. One fragment is engineered to be expressed in an animal test subject while the other fragment is injected into the animal’s circulatory system.
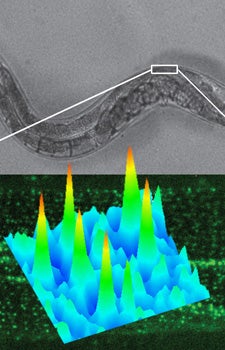
A new single-molecule imaging technique developed at USC Dornsife provides new insights into the role of dystrophin proteins for muscle function in Caenorhabditis elegans worm models of Duchenne muscular dystrophy. Graphic courtesy of Fabien Pinaud.
When they meet, the fragments unite and start emitting fluorescent light that can be detected with incredible accuracy, offering imaging precisions of around 20 nanometers. Conventional optical microscopy of living tissues can only achieve a 200 nanometer resolution at best. For scale, a sheet of paper is 100,000 nanometers thick.
“Now, for the first time, we can explore the basic principles of homeostatic controls and the molecular basis of diseases at the nanometer scale directly in intact animal models,” said Fabien Pinaud, assistant professor of molecular biology at USC Dornsife and lead researcher on the project.
Pinaud collaborated with scientists from the University Claude Bernard Lyon in France and the University of Würzburg in Germany.
“There are trillions of proteins at work on an infinitely small scale at every moment in an animal’s body. The ability to detect individual protein copies in their native tissue environment allows us to reveal their functional organization and their nanoscale molecular behaviors despite this astronomical complexity,” Pinaud said.
Next, Pinaud and his colleagues will focus on engineering other colors of split-fluorescent proteins to image the dynamics of individual ion channels at neuromuscular synapses within live worms.
“It so happens that the same calcium channels we studied in muscles also associate with nanometer-sized membrane domains at synapses where they modulate neuronal transmissions in both normal and disease conditions,” Pinaud said. Using multi-color CALM, his team and collaborators will probe how these tiny active zones of neurons are assembled and how they influence the function of calcium channels during neuron activation.
The new technology is an example of the convergence of science and engineering at USC Dornsife, where researchers from both fields collaborate to create the tools that make scientific and medical breakthroughs possible.
This research was funded by USC startup funds and the computational work was supported by the USC Center for High-Performance Computing and Communications.