‘Electric bacteria’ are ready for their close-up
Imagine you wanted to plug a device into an outlet on your wall, but you didn’t have a cord that reached all the way. Instead, all you had were short snippets of wire that, put together, weren’t enough to cover the distance between the device and the wall. Say you spread them out so they weren’t touching each other but traced a dotted line that spanned the whole distance. How would you overcome the gaps between the snippets to get electricity flowing?
According to scientist Moh El-Naggar, that’s a conundrum that the Shewanella oneidensis species of bacteria faces for its very existence. He and his team at the USC Dornsife College of Letters, Arts and Sciences think they’ve discovered how the bacteria have solved it.
These single-celled organisms are known as “electric bacteria” because transferring electrons from their cell interiors to the external surfaces where they live — such as rocks deep underneath the Earth’s surface — is fundamental to their survival. Similar to how humans transfer electrons to oxygen inhaled in the lungs, the bacteria must pass electrons to the outside world in order to “breathe.”
Over the past decade, El-Naggar and his interdisciplinary team have been studying how the bacteria are able to reach into their external environment and get charge to flow across long distances — at least “long” for microbes whose length is a hundred times shorter than the width of a human hair.
Their paper, published in the Proceedings of the National Academy of Sciences (PNAS), is the first to reveal the “molecular dance” that allows the bacteria to get this electronic job done without a continuous “wire.”
A lightbulb moment
El-Naggar first became interested in studying electric bacteria because of the potential to harness their power for new sustainable technologies. Capturing electrons from the bacteria on electrodes is the basis of emerging technologies like microbial fuel cells that generate clean energy.
Developing and refining these sophisticated applications “boils down to figuring out the basic physics of how electrons move in cells,” says El-Naggar, Dean’s Professor of Physics and Astronomy and professor of physics and astronomy, and chemistry.
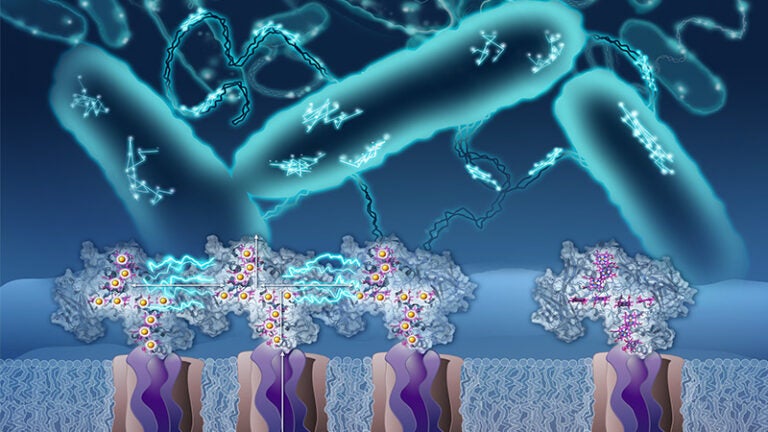
In a previous study published by PNAS 2018, he and his team showed that small, iron-containing proteins known as cytochromes carry electrons on the cell surface and along extensions of the cell membrane known as bacterial nanowires. But in order for these proteins to hand off electrons to one another, they have to be in pretty close proximity, which the team found wasn’t always the case. That raised the question: How would electricity flow between proteins that — like the snippets of wire in the outlet analogy — are not directly in contact?
El-Naggar hypothesized that the proteins may move around enough to collide with one another and transfer electrons, but confirming that guess would require seeing these microscopic molecules’ trajectories in real time — something that had never been done before.
Speaking at the USC Dornsife molecular and computational biology retreat in 2017, El-Naggar posed the question of how to observe the tiny proteins to his colleagues, with an eye on one colleague in particular: Fabien Pinaud, associate professor of biological sciences and physics and astronomy, whose lab has expertise in imaging single molecules.
“I said, ‘Wouldn’t it be nice if we could image this … hint, hint?’ and I looked straight at Fabien,” says El-Naggar. Pinaud took the bait and suggested that El-Naggar’s team figure out a way to attach quantum dots — which are crystals that emit light under the microscope, like miniature lightbulbs — to individual proteins. “This piece of insight that he gave right there enabled this whole project,” says El-Naggar.
Microscopic Moviemaking
Postdoctoral scholar Grace Chong ’21, who was a molecular and computational biology doctoral student in El-Naggar’s lab at the time, came up with what El-Naggar calls an ingenious solution for attaching the quantum dots. She engineered some of the proteins to express a tag that causes them to bind to the dots, like a socket to a lightbulb.
Chong viewed the modified cells under the powerful microscope in Pinaud’s lab. She used their single-particle tracking software to pinpoint the position of the quantum dots — which glow red when a laser is shined on them — at various timepoints and follow each protein’s trail.
To El-Naggar, the time-lapse “movies” Chong filmed of these red dots cruising across the surface of the cell membrane and bacterial nanowires make for incredible cinema.
“It’s a pretty mind-blowing thing,” he says. “It really felt like a privilege to be able to look at this process happening at the absolute limit of what we can observe, which is the movement of one individual molecule out of many millions that the cells are displaying at any given time.”
The protein dance captured in the movies confirmed El-Naggar’s original hypothesis. “For the first time,” says Chong, “we can see that [the proteins are] moving, that they’re colliding, that their tracks are overlapping with each other and tracing a path for long-distance electron transport along cells and membrane nanowires.”
[Story continues after video.]
Happy to see this study out now in @PNASNews. Our team tracked the movement of individual electron conduits on the cell surface and membrane nanowires of bacteria, using quantum dots. https://t.co/74iUEuU9f3 1/3 pic.twitter.com/3Ud0BRxG6i
— Moh El-Naggar (@BioPhysicalMoh) May 4, 2022
Power trip
Using the data Chong gathered, El-Naggar’s team conducted simulations to calculate how fast electrons move along the surface of a single cell or membrane nanowire. This rate has important implications for the development of new technologies that incorporate electric bacteria because it shows the maximum charge they can transmit.
El-Naggar notes that over the past few years, he’s become excited about the prospect of building “living” electronics, such as bacteria-based sensors that operate inside the human body or detect contamination in the environment.
“What I’m really thinking about are future applications that combine the best of the abiotic world — the world of metals and semiconductors — with the stuff biology is very good at,” he says, adding that as the bacteria demonstrate by moving electrons to meet their energy needs with a lot less “wire” than we might expect, “biology is very good at being efficient.”
About the Study
Additional authors on the study include former senior research associate at USC Dornsife Sahand Pirbadian and USC Dornsife PhD student Yunke Zhao. The study was supported by Department of Energy grant DE-FG02-13ER16415, Air Force Office of Scientific Research grant FA955014-1-029 and Office of Naval Research grant N00014-18-1-2632.