
Procreation trumps survival — even on a cellular level
A newly discovered biological mechanism channels a mother’s available energy — in the form of fat — straight to the reproductive system during stressful times, protecting future offspring at the cost of the mother’s health.
Sean Curran, assistant professor of biological sciences, observed the phenomenon in the worm species C. elegans, but the cellular mechanisms associated with it also exist in humans, raising the possibility that we may share this trait as well.
When an organism is exposed to external stresses such as famine, a protein that protects cells called SKN-1 is activated. In addition to stress resistance, activation of SKN-1 also drives the reallocation of lipids from the organism’s soma, or bodily cells, to its germline, or reproductive system, Curran found.
Once there, the fats fuel the development of oocytes, or egg cells, making successful reproduction easier; however, the animal itself faces a higher likelihood of a shortened lifespan. (Most C. elegans are hermaphrodites — Curran is still exploring whether the phenomenon also occurs with the male portion of the worm’s reproductive system.)

Sean Curran, assistant professor of biological sciences at USC Dornsife. Photo courtesy of USC Davis School of Gerontology.
When the organism again obtains nutrients, the presence of omega-3 and omega-6 fatty acids stop the travel of fats into the reproductive cells, bringing the animal’s ability to resist environmental stressors back to normal.
“SKN-1 plays essential roles in survival to stress at all stages in life; however, SKN-1 activation mutants are not long-lived. This is incredibly surprising and confusing at the same time since these animals should be stress resistant,” said Curran, who also is assistant professor at the USC Davis School of Gerontology. “Our study shows that the reason constitutively active SKN-1 doesn’t confer longevity is because of the movement of lipids from the soma to the germline to promote the necessity of reproduction.”
Oxidative stress
Curran is the senior author of a study on the mechanism published by Proceedings of the National Academy of Sciences. The research team included USC Dornsife graduate students Dana Lynn and Hans Dalton, as well as scientists from USC, Baylor College of Medicine and Massachusetts General Hospital. The researchers looked at stored fat molecules within the worms by staining cells and by using gas chromatography and mass spectrometry. The animals underwent stress from starvation and calorie restriction as well as oxidative stress from hydrogen peroxide exposure.
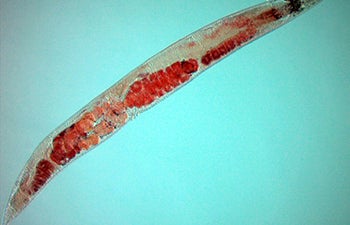
Red dye marks lipids in the soma of a worm. Shown here, dye is concentrated in the germline of a worm under stress. Photo courtesy of Sean Curran.
Oxidative stresses — an overload of reactive oxygen molecules that has been associated with cancer — can also trigger the activation of SKN-1. Since all organisms that require oxygen have to respond to oxidative stress, an understanding of how oxidative stress responses impact reproduction and vice versa is likely to yield more insights into how survival and reproduction balance against each other depending on resource availability, Curran said.
“This is particularly important in the wild, where resource availability is highly variable, and unlike in the laboratory, animals in nature must constantly assess possible risk and future reward,” he explained.
Curran is subsequently exploring the signaling mechanisms underlying this fat reallocation and the environmental triggers of the response. Since everything his team has found so far that regulates SKN-1 in worms has also been identified in humans and the balance of somatic resistance and reproduction is important for all organisms, the findings could have implications for reproductive success in older humans, he said.
Support for the work was provided by the National Institutes of Health (grant T32AG000037 to first author and Molecular Biology PhD student Dana Lynn, R00AG032308 and R01 GM109028 to Curran), the American Heart Association (Curran), an Ellison New Scholar Award (Curran) and the American Federation for Aging Research (Curran).